What Even Is a Buffer? Mobile Phase Considerations
What Is a Buffer Solution?
A buffer is a solution that is able to maintain its pH when an acid or a base is added to it in small amounts. The solution itself is made up of an acid and its conjugate base or vice versa.
When solutions are mixed with a very strong acid or base the pH will change rapidly and dramatically – this can be dangerous. A buffer solution will neutralize the acid or base that has been added in order for the pH to change more slowly – offering a more controlled reaction.
A buffer solution consists of components that react with either the hydrogen or the hydroxide ions which blocks them from affecting the pH at their full potential.
However, buffer solutions have a limit of how much they are able to neutralize before the solution reaches its capacity. Once the buffer solution is at its maximum capacity the pH of the solution will begin to change as if there was no buffer present at all.
What Are They Used For?
Buffers are incredibly useful solutions in chemistry and are used in many different types of reaction and industry. For example, in the fabric dyeing industry they are used to maintain the correct pH for the dye, they are also used in the production of alcohol – to keep the pH at a level that inhibits acidity.
Buffer solutions are also used to calibrate pH meters and in mobile phases to control pH.
What Are The Components of a Buffer Solution?
Acidic buffers (which have a pH of less than 7) are made with a weak acid and its conjugate base (a salt). Changing the pH of the solution is possible by changing the ratio of the weak acid and the salt. The acid itself also has an effect – different acids will affect pH in different ways.
Basic buffers (which have a pH of greater than 7) are made from a weak base and its conjugate salt – as with acidic buffer you can change the ratio of the weak base and its conjugate salt in order to change the pH.
What Happens When You Add Acid To a Basic Buffer?
![]()
Figure 1: Example of an alkaline buffer solution.
Figure 1 shows an example of a typical basic buffer-acid reaction – the hydrogen (from the acid) would interact with the ammonia to form ammonium ions which causes the removal of hydrogens. The ammonium ion is weakly acidic meaning it will release some hydrogen ions back into solution.
Hydrogen from the acid will react with hydroxide ions in solution to form water. The equilibrium will change to favour the formation of hydroxide ions, and this continues until the majority of the hydrogen ions are removed.
Subsequently by these methods the solution will change pH due to the removal of the majority of the hydrogen ions and therefore the acidic presence.
What Happens When You Add a Base To a Basic Buffer?
During a buffer-base reaction (example in Figure 1) – the buffer will be trying to remove the hydroxide ions from the added base and the ammonium ions in the solutions with react with those hydroxide ions. The ammonia that is formed is a weak base and it can react with water which means that the reaction is reversible. This means that the majority of hydroxide ions are removed from solutions and the pH change is minimal.
Henderson-Hasselbalch Equation
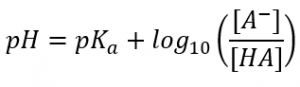
Figure 2: The Henderson-Hasselbalch equation.
The Henderson-Hasselbalch equation (shown in Figure 2) uses the pKa of the weak acid to calculate the pH of the buffer solution.
Buffers and HPLC
Why is it important to control pH in HPLC mobile phases?
When ionizable compounds are present the pH may need to be controlled – if the mobile phase pH is near the pKa small changes in pH can massively affect the retention time. Small changes in mobile phase pH can also affect separation (selectivity). The pH of a mobile phase is very susceptible to change and can even change overnight due to the evaporation of volatile acids or the dissolution of carbon dioxide into the solution.
Understanding your analyte is important when choosing a buffer as ion-suppressed analytes will retain better than ionized analytes.
pH and The Column
Another factor to consider when choosing the pH of a mobile phase is the stability of the column – generally speaking silica-based columns should be operated at a pH of 2<pH<8. A pH of less than 2 may cause bonded phase loss due to the hydrolysis that can occur. Silica that is of good quality and high purity will generally tolerate higher pHs better than a lower purity silica. Always refer to the column certificate of analysis for the pH tolerance of the analytical column.
The potential ionization of silanols on the silica surface of the column can also affect the retention of acidic and basic analytes. Usually, silanols will be deprotonated I.e., negatively charged. Due to this it is likely that positively charged, basic ions, will have greater retention, this can lead to an ion exchange interaction. This secondary interaction often results in peak broadening or peak tailing.
Buffers and UV
Some buffers may also interfere with UV detection; therefore, it is important to know the UV cut-off of the mobile phase additive. Mobile phases which have a UV cut-off below the detection wavelength will not compromise signal sensitivity.

Figure 3: A pH meter.
Buffer Selection
Choosing the appropriate buffer is regulated by the buffer’s characteristics such as pKa and pH range. The pH of the mobile phase will depend upon the analyte pKa value – so a buffer should be chosen that has a pKa value that is close to the pH value of the required mobile phase, generally within +/-1 units.
If using mass as a detector, the choice of buffer should also be carefully considered. Inorganic buffer salts such as phosphate salts will dirty the system, producing poor results. Therefore, it is paramount that only volatile buffers, such as formic acid and ammonium acetate, are used when working with a mass spectrometer.
The concentration of the buffer solution should be the lowest concentration that gives reproducible results. The higher the concentration of the buffer the faster polar compounds will be eluted. If the concentration of buffer is too low the additive may not behave as a buffer, and if the concentration is too high, the risk of buffer precipitation is increased which can cause column back pressure to build.
Download PDF
Get a PDF copy of this technical note here.
For more information or assistance, please get in touch.
