What are the common ionization methods for GC/MS
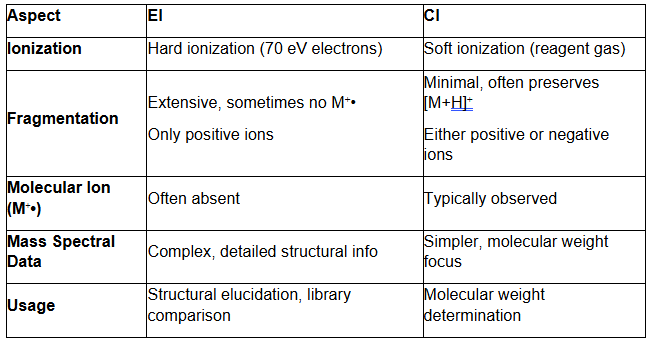
In gas chromatography-mass spectrometry (GC/MS), two common ionization methods are Electron Ionization (EI) and Chemical Ionization (CI).
Electron Ionization (EI)
Technique:
In Electron Ionization, high-energy electrons (typically 70 eV) bombard gas-phase analyte molecules. This collision strips an electron from the molecules, producing a positively charged molecular ion (M⁺•) and often causing fragmentation into smaller ions.
Fragmentation:
As a hard ionization method, EI delivers enough energy to break molecular bonds, resulting in extensive fragmentation. These detailed fragmentation patterns help identify molecular structures and build spectral libraries.
Advantages of EI:
- EI ionizes over 90% of compounds suitable for GC analysis, making it a universal method.
- It generates highly reproducible fragmentation patterns, which aids in compound identification.
- Many commercial mass spectral libraries, like NIST and Wiley, use EI spectra for easy compound matching.
- It identifies components in unresolved chromatographic peaks and provides quantitative data.
Limitations of EI:
- Extensive fragmentation can obscure the molecular ion (M⁺•), making it challenging to determine molecular weight.
When to Use Electron Ionization:
- When you need detailed structural information due to fragmentation.
- Quantitative analysis in complex matrix
- Identify unknown components by comparing spectra against standard libraries.
- Ideal for small, volatile, semi-volatile and thermally stable compounds.
Chemical Ionization (CI)
Technique:
Chemical Ionization uses a reagent gas (such as methane, isobutane, or ammonia), which gets ionized by electron bombardment. The ionized reagent gas then reacts with the analyte, forming a protonated molecule ([M+H]⁺) or other adducts instead of fragmenting the analyte.
Fragmentation:
As a soft ionization technique, CI causes minimal fragmentation and typically preserves the molecular ion or generates quasi-molecular ions like [M+H]⁺.
Types of CI:
- Positive Chemical Ionization (PCI): Produces pseudo-molecular ions with minimal fragmentation but generally offers lower sensitivity than EI.
- Negative Chemical Ionization (NCI): Provides very soft ionization, selectively detecting molecular ions. NCI offers high sensitivity for compounds with electronegative elements.
Advantages of CI:
- Preserves the molecular ion, making molecular weight determination easier.
- Provides complementary information to EI.
- NCI offers high selectivity and sensitivity, similar to an ECD detector, for specific compounds.
Disadvantages of CI:
- Reduced fragmentation limits the ability to deduce structural information.
- CI spectra depend on variables like reagent gas type, pressure, and temperature, limiting the availability of standardized spectral libraries.
When to Use Chemical Ionization:
- When molecular weight information is critical, and you want to preserve the molecular ion Especially for compounds that undergo excessive fragmentation or where the molecular ion is not observed in EI.
- NCI can be used for analysing high electronegative compounds, such as halogenated species and alkylating agents , where very low detection limits are required.
Summary of Differences between Electron Ionization and Chemical Ionization:
Choosing EI or CI:
Choose Electron Ionization (EI) when you need detailed spectral information and high sensitivity for quantitative analysis of target compounds. EI also works best for identifying unknown compounds by matching their mass spectra against existing spectral libraries.
Opt for Chemical Ionization (CI) when determining molecular weight is essential, especially if the molecular ion does not appear in EI.
You can use both methods complementarily in GC/MS analysis, depending on your specific analytical needs.