Importance of using analytical standards – qualitative and quantitative analysis
What is analyte confirmation?
Analyte confirmation can be specified as a requirement for method validation to ensure that compounds during the analysis have been correctly identified. This can be important across many industries to ensure active ingredient(s) and/ or impurities within a sample can be validated as the purity or identity they have been described as for both safety and quality control.
Method validation is crucial to ensure that the method being used is reliable, accurate and precise and therefore suitable for your analysis.
What are analytical standards?
Analytical standards are reference materials used for qualitative and quantitative analysis. They are fundamental in scientific research and industry to allow accurate and reliable results. Analytical reference standards have a known purity, concentration or composition and are used as a trustworthy reference within laboratories for instrument calibration, method validation, quality control and regulatory compliance.
When would you use analytical standards for analyte confirmation?
Analyte confirmation is commonly conducted using a highly selective detector such as a mass spectrometer (MS) for gas chromatography and liquid chromatography (GC-MS and LC-MS). Analyte confirmation can be carried out using analytical reference standards when a non-selective detector is being used such as a flame ionisation detector (FID).
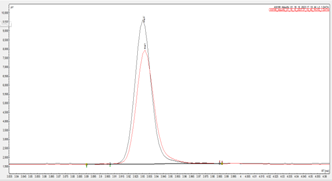
For qualitative analysis, the retention time of an analytical standard can be compared to that of unknown peaks to assign the identity of an analyte, see Figure 1. For non-MS analyte confirmation, this can be verified by using a different chromatographic technique such as an alternative phase GC column. For example, analysis can initially be run on a SCION-5 phase column and then confirmation performed using a SCION-WAX phase column. This is known as complimentary chromatography, see our technical note for more information.
Whilst using the NIST library can mean a confident identification of analytes, for true confirmation using a MS the use of an analytical standard is still required. An analytical standard can be run and the retention times compared to the compound being analysed. An exact retention time match will confirm the analyte identity.
If the retention time is not an exact match it can mean that the compound being analysed is not what you think it is or the sample matrix could be interfering with the chromatography. To confirm the identity of the target analyte in a sample matrix, samples are prepared to the same method but some samples will be spiked with the known analytical standard. If the target analyte has been correctly identified then the results from the spiked samples would be expected to show a clear increase for the peak area with an exact match in retention time compared to the samples from your analysis for the target analyte.
The combination of the NIST spectral library match and confirmation using an analytical standard, means it can be concluded that the target analyte was identified correctly.
Choosing an analytical standard
When choosing an analytical standard for your analysis there are certain factors that must be taken into account such as purity, traceability, stability, concentration and certification. Depending on your laboratory or method being used you may need standards which are certified to a certain specifications or requirements depending on quality grades. Analytical standards can be costly so it is important to ensure you are purchasing the correct standard which is suitable for your analysis. Note that depending on dilutions involved in your method, ensure the concentration and amount purchased of the analytical standard is suitable for your application.
Figure 1 Overlay of eugenol sample against the impurity 3 analytical standard on SCION-5 column.
Best practice when using analytical standards
After purchasing analytical standards it is important to store and use them in accordance with the manufacturer guidelines e.g. store at a specific temperature or protected from sunlight. It is important to retain or acquire from the manufacturer website any documentation for the purchased standard such as a certificate of analysis (CoA). The CoA will state information such as lot number, purity and expiry dates. When preparing stocks and standard solutions using an analytical standard it is crucial to be confident of your dilutions and mix solutions well so the concentration of the solution is correct and consistent for your analysis.
Importance of using analytical standard for quantification
For quantitative analysis, the amount of analyte in your sample can be determined by the peak height or area under the peak. To be able to determine an analyte’s concentration a calibration curve can be created from known concentrations of an analyte in solution. During analysis, it is important to inject a series of solutions of known concentrations to gain the accuracy and precision of your analytical set up. The use of an internal standard is recommended to account for variance within your system. See our technical note on the Importance of using an Internal Standard.
A single point standard can be used for quantitative analysis by preparing a standard solution of a known concentration of the target analyte and measuring it’s signal then calculating the response. This approach is simple but if there is an error in the single measurement then that will carry across all calculations. This approach also assumes the response across the concentration range being calculated is constant which may not be the case. It is most desirable to create a calibration curve to determine the linearity which encompasses all concentrations being analysed.
In order to be confident of the concentration and identity of the analyte being injected, the use of an analytical standard is essential. Consistency when integrating peaks is key to successfully quantifying analytes correctly. See our technical note on Good Integration.
Identification of similar compounds using analytical standards (reference compounds)
Analytical reference standards can be used to compare and assign an unknown. It can be assumed that if a compound elutes near a known standard it will possess a similar chemistry to the known analyte and therefore should show a similar response factor which can be compared to the analytical standard.
This can be helpful as it can mean, for example, when analysing the impurities from a process that only one analytical standard needs to be purchased and it can be used as the reference compound for multiple close eluting analytes. The results can be used with reasonable confidence and costs of the application are kept to a minimum. An example of this can be seen in our application note on Plant Protection Products Impurity Screening by GC-FID with GC-MS Confirmation for the impurities of Eugenol.