An Alternative to Mass Spec (MS) Analyte Confirmation - Complementary Chromatography
What is analyte confirmation?
When validating an analytical method it is highly likely that analyte confirmation will be required for either active ingredients(s) and/or impurities present within the sample.
Analyte confirmation ensures confidence that we have correctly identified the target analytes of the method, this is required across a number of different industries from the pharmaceutical industry to the pesticide industry.
How is analyte confirmation performed?
Typically analyte confirmation is performed using highly specific methods such as GC-MS with a minimum of 3 ions or HPLC-MS/MS or GC-MS/MS with a minimum of 2 transitions or one transition with several respective fragments of the substance are typically used for identification.
Is analyte confirmation possible without mass spectrometry?
Analyte confirmation is also possible using a technique called complementary chromatography. Confirmation can be achieved by using a chromatographic technique which is different from the original, by using a different mobile phase or by utilizing a different column, for example.
Method validation using a SCION-5 column
A method was validated for impurities in a chemical product according to Plant Protection Products regulations (see our application note AN166 for full detail).
Figures 1 and 2 show examples chromatograms for 2 impurities found within the sample, both figures show an analytical standard of the impurity overlaid with the impurity peak found within the sample.
The match in retention time shows that it is highly likely the correct impurity has been identified.
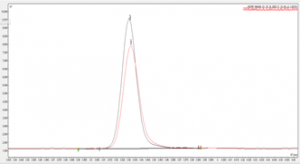
Figure 1. Overlay of eugenol sample against the impurity 3 analytical standard on SCION-5 column
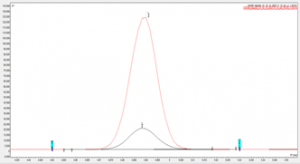
Figure 2. Overlay of eugenol sample against the impurity 7 analytical standard on SCION-5 column.
The particular application was following the SANCO guidance document for plant protection products (pesticides). This document requires analyte confirmation for any impurity species in order to be able to successfully submit a product.
SANCO gives several options for analyte confirmation including “GC-MS with a minimum of 3 ions, HPLC-MS/MS or GC-MS/MS with at least 2 transitions or one transition with several representative fragments of the substance”.1
Another option defined is complementary chromatography “confirmation can be achieved by an independent analytical method using for example a chromatographic technique different from the original, a different stationary phase and/or mobile phase with a significant different selectivity or a different detector”.1
Complementary chromatography was performed as analyte confirmation for this method as follows:
Complementary Chromatography of impurities in eugenol product using a different column phase (SCION-WAXMS)
An alternative column was chosen, the SCION-WAXMS which has a very different column chemistry to the SCION-5. The wax column is far more polar and presents a good option for analyte confirmation as any analytes will interact very differently than with the non-polar SCION-5.
Figures 3 and 4 show examples chromatograms for 2 impurities found within the sample, both figures show an analytical standard of the impurity overlaid with the impurity peak found within the sample.
The match in retention times between the standards and the sample across 2 different column chemistries shows confidence in the correct impurities having been identified, we have now satisfied the requirements for analyte confirmation.
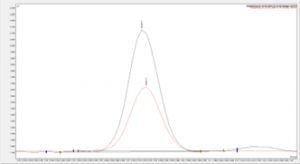
Figure 3. Overlay of eugenol sample against the impurity 3 analytical standard on SCION-WAX-MS column.
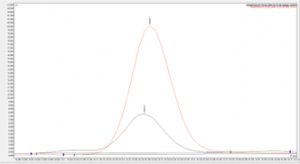
Figure 4. Overlay of eugenol sample against the impurity 7 analytical standard on SCION-WAX-MS column.
Note the concentration of the analytical standards were different from the SCION-5 and SCION-WAXMS chromatograms shown, which account for the differences seen in the peak height/area. As this is a qualitative analysis only this has no effect on the validity of the results.
Equally the same samples can be ran as it is the change in column phase or other method conditions that defines this test, and there is no requirement for fresh samples so long as relevant expiry dates are observed.
Considerations
Depending on the regulation being followed, analyte confirmation may require full validation of the secondary method being used for analyte chromatography.
For example NATA (National Association of Testing Authorities, Australia) requires full validation of any method following ISO 17025 and ISO 15189. Whereas the SANCO guidance document which is relevant for plant protection products (pesticides) in the EU requires just an analytical reference standard to be ran alongside a sample containing the relevant analytes.
Requirements will vary by industry and by region, SCION instruments recommends checking with local authorities before performing any testing to ensure compliance with local regulatory guidelines.
Complementary chromatography can be utilized on both GC and HPLC systems.
Impurity Screening – Unknowns
One possible drawback to this technique is that it can make identifying unknown impurities more difficult, whereas with an MS confirmation we are much more likely to be able to identify any unknowns using library searches such as NIST. See our application note AN165 for full detail on impurity screening for unknown compounds.
For samples where the impurity profile is well established and unlikely to change this presents the perfect opportunity to benefit from the technique of complimentary chromatography and negate the need for an MS.
References
1. European Commission, SANCO/3030/99 rev.5, 22 March 2019
Download Technical Note
Download a copy of this technical note using the following link – An Alternative to MS Analyte Confirmation
