Plant Protection Products Active Ingredient Assay by GC-FID with Nitrogen Carrier Gas
Introduction
An active ingredient (AI) is the component of a chemical product which produces the desired chemical or biological effect.
Determining AI content is a requirement across many regulated industries from pharmaceutical, food, agriculture, and environmental.
In this application note we will discover how to validate a method and assay for the AI content of a plant protection product (PPP).
Plant protection products (PPP) or pesticides are subject to strict regulations for import to countries all over the world.
In this application note we will look at EU and UK regulations for submission of technical active substances and PPP No 283/2013 (Annex Section 4) and 284/2013 (Annex Section 5). These regulations have a guidance document SANCO/3030/99 rev5[1] which sets out the testing required for a successful submission of a product.
This guidance document is also relevant for other global submissions such as US EPA Pesticide Assessment Guidelines OPPTS 830.1700 and 830.1800 as well as other countries such as Mexico, Brazil, Australia and Canada, however there are some differences in the testing or format of the report required. SCION instruments recommends checking with local authorities in your region of interest before performing testing.
The product chosen for testing in this application was a commercially available bottle of technical grade active ingredient (TGAI) Eugenol. Eugenol is a terpene which makes up around 80% of clove leaf oil and is attributed to the distinctive smell of cloves. Eugenol is also a powerful insecticide and when imported for this use falls under the requirements of the PPP regulations.[2]
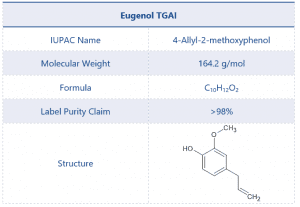
The chemical structure and other details of Eugenol can be found in Table 1.
Helium has long been the standard carrier gas for GC applications however, due to recent global shortages many companies are keen to find alternatives.[3] The application described in this note has been performed using an alternative carrier gas, nitrogen. This can be produced with generators using renewable energy sources and so is a cleaner, greener option as well as avoiding the current issues over helium supply.
Table 1 Test Item Details
This application can be performed on either the SCION Instruments 8300 GC or 8500 GC platforms equipped with an 8400 Pro Autosampler, S/SL injector, and FID detector. These are shown in Figure 1.


Figure 1 SCION Instruments 8300 GC and 8500 GC platforms equipped with 8400 PRO Autosampler
Experimental
Section 4.1.1. of the SANCO guidance document details the validation requirements of any method used to quantify the active ingredient of a product.
The document sets out strict requirements for specificity, linearity, recovery and precision of the method.
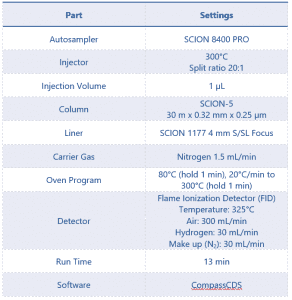
After developing the instrument method, the conditions of which can be seen in Table 2, the optimum concentration of the samples was determined. It was found that a nominal or target concentration of 0.5 mg/mL gave a suitable response for quantification of the active ingredient Eugenol.
A suitable internal standard was chosen, Hexadecane (purity 99.8%), which had a similar response factor to Eugenol and did not interfere with the active ingredient or any impurities present.
Table 2 Instrument Parameters
The internal standard solution was prepared by weighing 1g Hexadecane analytical standard into a 2L reagent bottle, this was made to volume using Acetonitrile (HPLC grade 99.8%) and mixed well. This solution was used to make all further samples to volume.
Linearity samples were prepared as per the guidance document at 5 concentrations ranging at least +/- 20% of the nominal concentration. 40, 45, 50, 55, and 60 mg of Eugenol TGAI was weighed into separate 100 mL volumetric flasks. These samples were made to volume with the internal standard solution. (Either the product or an analytical standard can be used for the validation of an active ingredient, often the TGAI product will be used as it is more readily available and cost effective).
Method precision samples were prepared as per the guidance document by weighing 50 mg of Eugenol TGAI into 5 separate 100 mL volumetric flasks, these samples were made to volume with the internal standard solution.
Recovery samples were prepared at 2 concentrations, 80 and 120% of the nominal. Samples were prepared at each level in triplicate by weighing 40 mg (80%) and 60 mg (120%) into separate 100 mL volumetric flasks, these samples were made to volume using the internal standard solution.
These validation samples were ran on a SCION 8500 GC according to the method conditions in table 2.
The quantification of the active ingredient content within the Eugenol TGAI was calculated using a Eugenol analytical standard (Purity 99.8%).
A reference standard was prepared by weighing 50 mg of the Eugenol analytical standard into a 100 mL volumetric flask, this sample was made to volume with internal standard solution. A quality control (QC) standard was also prepared to confirm the method was continuing to perform well, this was prepared in the same manner as the reference standard. Eugenol TGAI samples were prepared in triplicate by weighing 50 mg Eugenol TGAI into separate 100 mL volumetric flasks, these samples were made to volume using internal standard solution.
The Eugenol TGAI samples were ran, bracketed by the reference and QC standards, immediately after the validation samples.
If multiple batches of Eugenol were being submitted, each batch would be ran in triplicate, bracketed by reference and QC standards, the method validation would apply for each batch and would not require repeating for each individual batch.
Results and Discussion
The instrument method stated in Table 2 gave excellent specificity. Figures 2 – 7 show example chromatograms of a blank (diluent only), reference standard, and technical sample, as well as an expanded chromatogram of each showing the region of interest where Eugenol and the internal standard elute.
These chromatograms clearly demonstrate the specificity of the method, showing excellent resolution between all the components as well as good peak shape of both Eugenol and the internal standard.
SANCO defines the criteria for acceptable linearity as a correlation coefficient (r2) value as >0.99. Table 3 shows the linearity results for Eugenol.
Table 3 Linearity results for Eugenol (calculated using the peak area ratio of Eugenol/Internal standard)
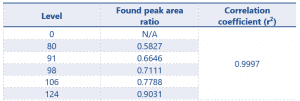
The r2 value of 0.9997 demonstrates excellent linearity, greatly exceeding the criteria defined by the SANCO document.
Figure 2 Example Chromatogram of a blank sample (Acetonitrile only)
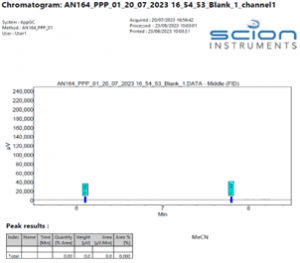
Figure 3 Expanded Chromatogram of a blank sample (Acetonitrile only)
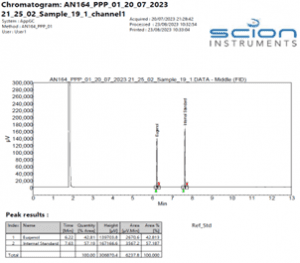
Figure 4 Example Chromatogram of a Eugenol reference standard
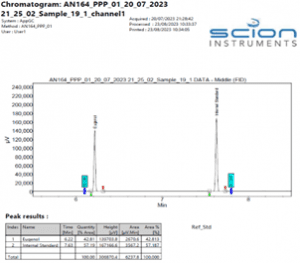
Figure 5 Expanded Chromatogram of a Eugenol reference standard
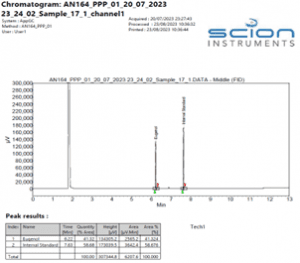
Figure 6 Expanded Chromatogram of a Eugenol TGAI sample
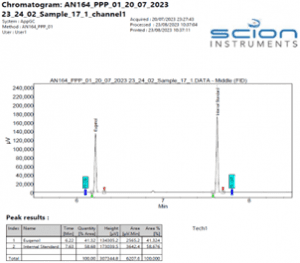
Figure 7 Expanded Chromatogram of a Eugenol TGAI sample
Appendix 2 of the SANCO guidance document details the criteria for validation results. For recovery any active substance or impurity present at ≥10% requires a mean recovery of 97 – 103% to be considered acceptable.
Precision results are determined to be acceptable using the Horwitz equation. The Horwitz equation corresponds to an exponential relationship between the estimated intra-laboratory repeatability (RSDr) and concentration (c).
1):
![]()
For an active ingredient with expected content of 95%+ we will use the %RSDr for 100% w/w. This gives us a value of 1.34 for %RSDr or the Horwitz value.
With this obtained value for the Horwitz value the Horwitz ratio (Hr) can be calculated, using the obtained repeatability (%RSD) of the target component.
2):
![]()
From this calculated value (Hr) the following criteria are applied:
Hr ≤ 1, acceptable.
1 < Hr ≤ 2, acceptable in case of a suggested explanation.
Hr > 2, not acceptable.
Method precision results can be found in Table 4.
Table 4 Method Precision results for Eugenol
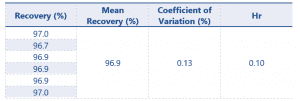
The results for method precision are excellent with a Hr value well below the criteria defined by SANCO.
Assay accuracy and precision results are shown in Table 5, this shows the combined recovery and coefficient of variation for both prepared concentrations (80 and 120% w/w).
Table 5 Assay Accuracy and Precision results for Eugenol

The results for assay accuracy and precision also easily pass the criteria set by SANCO. With these results the AI method can now be considered fully validated and the content of Eugenol present in the product can be accurately quantified.
The results of the Eugenol assay can be found in Table 6.
Table 6 Results for Eugenol content
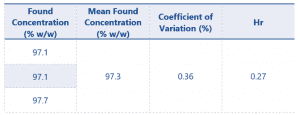
The 3 replicates of the Eugenol product gave a mean found concentration of 97.3% w/w. The Horwitz ratio of 0.27 between the 3 samples shows great reproducibility, easily meeting the criteria defined within the SANCO document.
The QC standard was determined to have a recovery of 99.2% w/w which confirms the correct working of the AI method during the determination of Eugenol content in the product.
The Eugenol product used for this method validation and assay had a purity claim of >98%. This product had been imported under the much less stringent REACH regulations and was not intended for use as a PPP.
However, this result still demonstrates the importance of regulatory testing using fully validated methods to ensure the quality of products entering the market, as the purity can vary greatly from the label claim.
The EU defines the minimum purity required for Eugenol to be used as a PPP to be ≥99.0% w/w.
Conclusion
Active ingredient (AI) content is a requirement of testing across several regulated industries. In this application note we have seen how to fully validate and assay for an active ingredient (Eugenol) in compliance with the guidance document SANCO/3030/99 rev.5 for submission of a plant protection product or pesticide within the EU or UK.
This testing would also form the basis for submission to multiple other countries such as the US, Mexico, Brazil, Canada, and Australia. SCION instruments recommends checking with local regulatory authorities to ensure all testing and reporting requirements are met, or contact the SCION applications team for assistance.
The principles used in this application note can be applied to not only insecticides, but to other environmental pesticidal products such as biocides and fungicides as well as across other industries such as cosmetics, food, and drug testing.
The product tested in this application note (Eugenol) was shown to fall below it’s label claim of 98%. With the AI assay giving a purity value of 97.3%. This product was imported under less stringent (REACH) regulations and was not intended for use as a PPP. However, this result highlights the importance of rigorous regulatory testing to ensure the quality of products being imported.
Ordering Information
For ordering info on the SCION 8500 GC, which offers greater functionality with the option of up to 4 detectors (including MS), please contact your local SCION sales representative
References
1. European Commission SANCO/3030/99 rev.5, 22 March 2019 https://food.ec.europa.eu/system/files/2019-03/pesticides_ppp_app-proc_guide_phys-chem-ana_3030.pdf
2. Commission implementing regulation (EU) No 546/2013, Approval of Eugenol for use as a PPP, 14 June 2013 lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:163:0017:0020:EN:PDF
3. Innovation News Network, Helium Shortage 4.0, April 04, 2023 https://www.innovationnewsnetwork.com/helium-shortage-4-0-what-caused-it-and-when-will-it-end/29255/
Download Application Note
Download the PDF version of this Application Note: Plant Protection Products Active Ingredient Assay by GC-FID with Nitrogen Carrier Gas
