Import and Export of Chemicals EU/UK REACH Substance Identity Requirements
Introduction
REACH stands for the registration, evaluation, authorisation and restriction of chemicals. It is a European regulation which dates from December 2006. Manufacturers and importers are required to gather information on their chemicals and then register that information in the European chemicals agency database.
In 2021 the UK REACH regulation came into force after the UK left the EU and the EU REACH regulation was brought into UK law.
UK and EU REACH regulations apply to almost all chemical substances that are either produced or imported into the UK – this may be a substance on its own, a substance existing in a mixture such as ink or paint, or a substance within an article – which is an object produced with a special shape, surface or design such as a car or furniture.1
REACH regulations aim to protect human health and the environment from harmful chemicals, ensure that the manufacturers and importers of chemicals understand and manage the risk associated with their chemicals and to promote alternative methods of assessment of hazardous properties of substances e.g. quantitative structure-activity relationships.1
Each different substance will have its own substance information and registration dossier defined by the European Chemicals Agency (ECHA).
This information is readily available online through ECHA’s website. Each profile provides boundary compositions which define the purity required for each substance and any relevant impurities that need to be tested for a successful submission. Often a registration dossier will provide multiple boundary compositions for a single substance, this will depend on the number of different uses of a substance, with the required specifications varying based on the use and risks associated with that use.
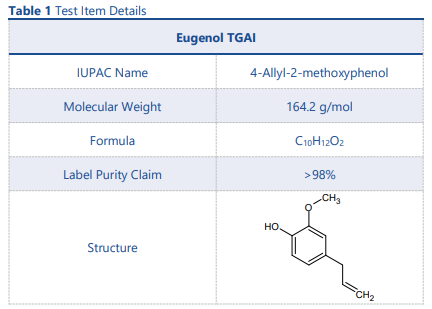
The test item chosen for this application note was a commercially available bottle of technical grade active ingredient (TGAI) eugenol. Eugenol is a terpene which makes up around 80% of clove leaf oil and is attributed to the distinctive smell of cloves. The chemical structure and other details of Eugenol can be found in Table 1.
Eugenol is imported for multiple uses which fall under the REACH regulation.2
Experimental
For a successful submission of a substance under the REACH regulation we are required to assess the purity of the substance and to confirm the active ingredient(s) or main constituent(s) identity.
For eugenol the purity was assessed using gas chromatography equipped with a flame ionization detector (GC-FID) and the identity of the active ingredient confirmed using a GC equipped with a single quadrupole mass spectrometer (GC-MS).
For the purity determination of the eugenol sample it is important to chose a concentration of the test item so that the main constituent is on scale but also so that any significant impurities present are easily detected.
A nominal concentration of 10 mg/mL with regards to the technical material was chosen due to the suitable response shown by the impurities at this concentration.
A technical sample was prepared by weighing 100 mg eugenol TGAI (CoA claim 98% purity) into a 10 mL volumetric flask, the sample was made to volume with acetonitrile (HPLC grade, 98%) and mixed well.
Acetonitrile blanks were ran to confirm that any impurities detected in the eugenol sample were not caused by impurities in the solvent.
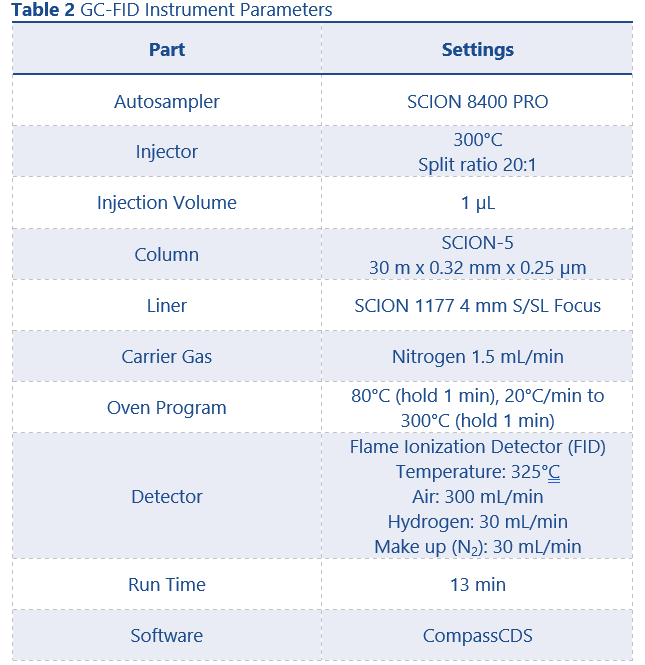
The technical sample was ran on an 8500 GC equipped with split/splitless (S/SL) injector and a flame ionization detector (FID). Method conditions can be found in Table 2.
For analyte confirmation of the active ingredient a eugenol sample was prepared at 50 µg/mL in Acetonitrile and ran on an 8500 GC equipped with 8700 SQ MS.
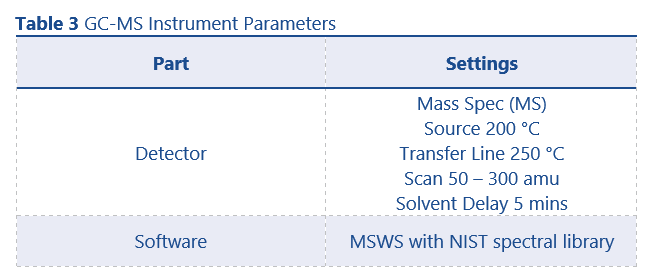
GC conditions were as per the method used for purity determination (with the exceptions that helium was used as carrier gas for GC-MS and the column was replaced with a SCION-5MS (30m x 0.25 mm x 0.25 µm)) and can be found in Table 2. MS conditions can be found in Table 3.
Results
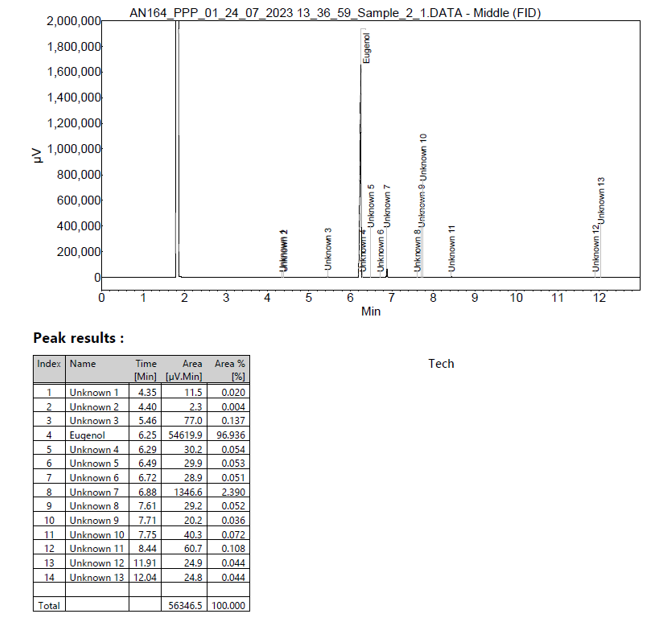
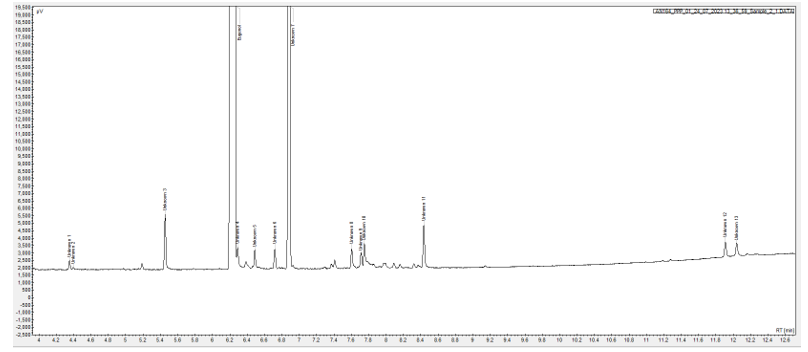
From the GC-FID run the purity of the sample was determined to be 96.94% by peak area %. 13 impurities were identified.
A chromatogram of the eugenol sample along with peak result Report can be found in Figure 1.
Figure 1 Chromatogram of 10 mg/mL eugenol sample and peak result report.
An expanded chromatogram showing the impurity profile can be seen in Figure 2.
Figure 2 Expanded chromatogram.
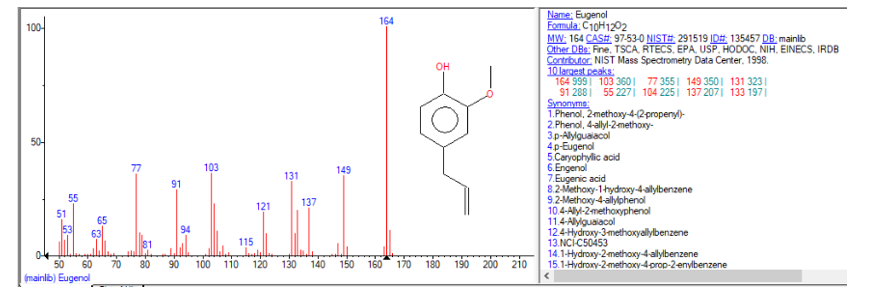
Analyte confirmation of the sample confirmed eugenol to be the main component. Confirmation was performed through the NIST library search function built into SCION’s MSWS software, an example screenshot can be seen in Figure 3.
Figure 3 NIST Library search confirming identity of eugenol
Conclusion
Testing was completed on a commercially available sample of TGAI eugenol. Purity of the sample was determined by GC-FID and was found to be 96.94% with 13 impurities were identified.
The substance identification and quantification requirement of the REACH regulation is less stringent than other regulations such as those governing pharmaceuticals, food or environmental (pesticides etc). Therefore there is no need to purchase analytical standards to accurately quantify components, with a simple relative % area report sufficing.
If ECHA defines a relevant impurity, likely a toxic impurity then it is advised to screen for and if necessary quantify any such impurities using analytical reference materials. See our application note, found on the SCION website, on impurity screening in plant protection products for more details on impurities and screening processes (AN165).
MS confirmation of the active ingredient was carried out using the powerful NIST database search tool incorporated into SCION’s MSWS software.
REACH requirements will vary from substance to substance depending on the type, composition and also the intended use of the substance.
Therefore it is important to understand not only the substance but also the end destination and market involved. Information can then be gathered from the ECHA website which will determine (through the appropriate boundary composition) the purity requirements and any relevant impurities that may need to be screened for.
Ordering Information
For ordering info on the SCION 8500 GC, which offers greater functionality with the option of up to 4 detectors (including MS), please contact your local SCION sales representative
References
1. UK REACH Explained, https://www.hse.gov.uk/reach/about.htm, (accessed May 2024)
2. ECHA Registration Dossier Eugenol, https://echa.europa.eu/registration-dossier/-/registered-dossier/13694/1/1, (accessed May 2024)
For more information, please contact:
E: sales-eu@scioninstruments.com
W: www.scioninstruments.com
Download PDF – AN175v1 Import and Export of Chemicals EUUK REACH Substance Identity Requirements