Sample Preparation – Liquid-Liquid Extraction
Why is liquid liquid extraction important?
Liquid-Liquid Extraction (LLE) is the exchange of compounds between two immiscible solvents. Typically, LLE is performed using an aqueous phase, such as water and an organic phase. The phase in which the analyte is contained depends upon the unique characteristics of the analyte itself. Where the analyte resides can also depend upon the nature of the aqueous phase.
Liquid-liquid extraction is used across many industries and is an important extraction method in research and chemical analysis.
The Target Analyte
In order to be able to understand and optimize LLE the physicochemical information of the target analyte must be understood. To achieve the optimal selectivity and recovery the partitioning of the analyte from one phase into the other needs to be efficient. The physicochemical properties of the target analyte will likely have the biggest effect on this. Parameters such as the pKa and LogP are the most essential ones to consider.
Which liquid-liquid extraction method is best?
The principle decision in designing any LLE experiment is the choice of extraction solvent – this is defined by the relative hydrophobicity of the target analyte, the LogP value of the analyte provides an indication of this.
The LogP value of the target analyte tells us the partitioning value between the organic and aqueous phases. An analyte with a highly positive LogP value will partition into the organic phase to a greater extent than an analyte with a more negative LogP value.
Ionogenic analytes will partition from the aqueous to organic phases when the analyte is in its neutral form. For acidic analytes the aqueous sample is typically adjusted to two pH units below the pKa value of the analyte and for basic analytes the pH should be adjusted to two pH units above the pKa.
For polar analytes an organic solvent with a higher polarity index should be chosen to ensure the maximised recovery of the target analyte from the aqueous sample – LogP can be used as a good indication of polarity.
In order to increase the partition coefficient of hydrophilic analytes simple salts can be used to saturate the aqueous
sample solution and increase the partition coefficient of the hydrophilic analytes.
Extraction Procedure
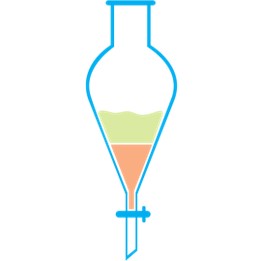
Most simply, LLE is taking two immiscible phases, one of which is aqueous and the other organic. The two phases are mixed together (often in a separatory funnel), the compounds within the sample will then distribute themselves between the two different phases. The liquids can then separated by filtering and the organic phase is often washed.
For high-throughput applications liquid-liquid extraction can be achieved by help of a liquid handling robot such as a Tom-Tec.
What is liquid liquid extraction used for?
LLE is perhaps one of the oldest and most widely used sample extraction techniques, it has applications across many different industries including pharmaceutical, pesticide and forensics.
LLE can also be used in the environmental and food industries for numerous applications – such as the analysis of semi-volatile organic compounds.
See our website for the application note on the analysis of semi-volatile organic compounds in drinking water by GC-MS.
LLE can be used on a variety of different sample matrices such as blood, urine, plasma as well as gastric contents, making it a highly versatile technique.
If you enjoyed this article, you might want to take a look at these other interesting reads:
https://scioninstruments.com/blog/sample-preparation-derivatization-extraction/
https://scioninstruments.com/blog/sample-preparation-manual-solid-phase-extraction/
https://scioninstruments.com/blog/sample-preparation-choosing-a-sample-preparation-technique/
