Plant Protection Products Impurity Screening by GC-FID with GC-MS Confirmation
Introduction
Impurities are an inevitable part of a chemical product; these are often process related, a by product of the synthesis or production of a product, or can be caused by degradation of the active ingredient (AI) or other components present within the product.
Determining impurity content is a requirement across many regulated industries from pharmaceutical, food, agriculture, and environmental.
But how do we decide which impurities are present within a product? Often chemical products can be very complex with dozens of impurities present, do we need to measure them all, or can we discount some, how do we decide this?
In this application note we will discuss how to determine which impurities should be identified and accurately quantified to satisfy regulatory requirements.
Regulations will vary by product type and by region, guidance documents will be available which will define the acceptable levels . SCION instruments recommends checking with local authorities in your region of interest before performing testing.
In this application note we will look at EU and UK regulations for submission of technical active substances and PPP No 283/2013 (Annex Section 4) and 284/2013 (Annex Section 5). These regulations have a guidance document SANCO/3030/99 rev51 which sets out the testing required for a successful submission of a product.
The product chosen for testing in this application was a commercially available bottle of technical grade active ingredient (TGAI) eugenol. Eugenol is a terpene which makes up around 80% of clove leaf oil and is attributed to the distinctive smell of cloves. Eugenol is also a powerful insecticide and when imported for this use falls under the requirements of the PPP regulations.2
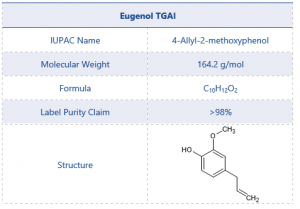
The chemical structure and other details of eugenol can be found in Table 1.
There are two distinct types of impurity that must be considered. Relevant impurities which are defined as being “impurities of toxicological and/or ecotoxicological or environmental concern”. These are defined by the product, for example eugenol, when submitted for use as a PPP, UK and EU regulations define methyl eugenol as a relevant impurity that can be present at a maximum of 0.1% of the technical material.3
The other type of impurity to consider is significant impurities. These are impurities that are present at or above a % defined by the relevant regulation. So for example for PPP products in the EU/UK the SANCO guidance document dictates that any impurity >0.1%.
Relevant impurities should be included in any impurity assay with a limit of quantification (LOQ) ≤ the defined regulatory maximum, even if they are not found in the technical material.
Significant impurities are a little trickier, as these are often unknowns. Firstly we need to determine which impurities require identification and quantification. The simplest way to go about this is to use an analytical standard of the active ingredient – in this case eugenol. We can assume that any impurities present within the technical material are likely to be very similar in nature to eugenol. Therefore we can perform a screening of all present impurity peaks and compare this to a 0.1% Eugenol standard (impurity limit defined by SANCO).
Once we have these results we are then able to exclude any impurities that have been calculated to be <0.1%. The remaining impurities >0.1% will then need to be identified using mass spectrometry (MS) and finally their identity confirmed using the relevant analytical reference materials.
Table 1 Test Item Details
Impurity Screening
A nominal concentration of 10 mg/mL with regards to the technical material was chosen due to the suitable response shown by the impurities at this concentration.
A technical sample was prepared by weighing 100 mg eugenol TGAI (CoA claim 98% purity) into a 10 mL volumetric flask, the sample was made to volume with acetonitrile (HPLC grade, 98%) and mixed well.
Acetonitrile blanks were ran to confirm that any impurities detected in the eugenol sample were not caused by impurities in the solvent.
A eugenol standard stock was prepared by weighing 50 mg eugenol analytical reference material (purity 99.8%) into a 100 mL volumetric flask, sample was made to volume with acetonitrile and mixed well. A dilution was then performed by pipetting 0.5 mL of this stock into a 25 mL volumetric flask, sample was made to volume with acetonitrile and mixed well to give 0.1% eugenol standard.
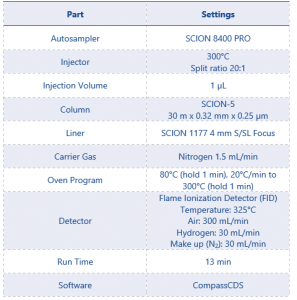
The technical sample was ran in duplicate, bracketed by the 0.1% standard, on an 8500 GC equipped with split/splitless (S/SL) injector and a flame ionization detector (FID). Method conditions can be found in table 2.
Table 2 GC-FID Instrument Parameters
13 potential significant unknowns had been identified using this method. These unknowns were quantified using the average of the standard injections. Results can be see in Table 3.
The results show 3 impurities as being present ≥0.1%, unknowns 3, 7, and 11. These 3 unknowns will now need to be identified using MS.
An example chromatogram of the eugenol TGAI sample can be seen in Figure 1.
Table 3 Screening Results for Eugenol TGAI
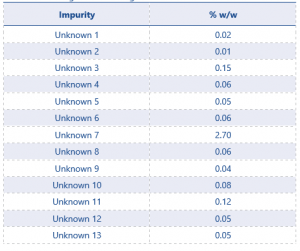
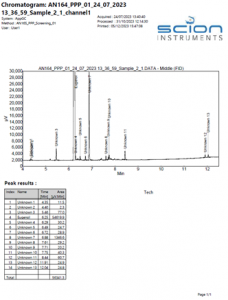
Figure 1 Example Chromatogram of a eugenol TGAI sample
MS Identification of Unknowns
In order to identify the unknown compounds a diluted eugenol TGAI sample (1 mg/mL) was ran on an 8500 GC with 8700 MS single quad (SQ). Method conditions can be found in Table 4.
Table 4 GC-MS Instrument Parameters
Using the powerful NIST spectral library search tool the MSWS software identified the most likely matches for each of the unknowns. The list of identified compounds can be seen in Table 5. For ease the 3 identified impurities shall be referred to as impurity 1, 2, and 3.
Example MSWS reports can be seen in Figures 2 and 3 for two of the unknown compounds.
Table 5 Identified Impurities

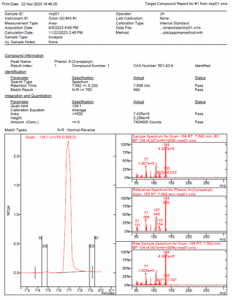
Figure 2 MSWS Report for Unknown 3
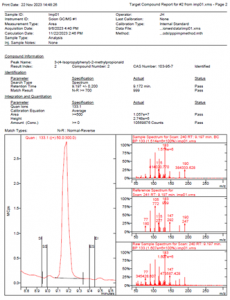
Figure 3 MSWS Report for Unknown 7
Confirmation Using Analytical Standards
Analytical reference standards of the identified impurities were sourced in order to confirm the correct identity of each impurity. Once the identity of each impurity has been confirmed these standards can then be used in the validation and assay of an impurity method in order to support the successful submission of the product to the relevant regulatory bodies.
3-(4-hydroxy-3-methoxyphenyl)-2-propenal was unable to be sourced at the time of testing from a reliable and cost effective source. Therefore only the other 2 remaining impurities were sourced.
The EU defines the minimum purity required for Eugenol to be used as a PPP to be ≥99.0% w/w. This particular product was imported under REACH regulations and could not be used as a PPP. We will therefore use the two impurities sourced as an example of analyte confirmation for a PPP.
In order to confirm the identities of the 2 impurities a eugenol TGAI sample (prepared at 10 mg/mL in acetonitrile) was ran under the conditions described in Table 2. Fortified eugenol TGAI samples were also prepared (10 mg/mL in acetonitrile) and spiked with the relevant impurity (0.1% unknown 3 and 1.4% unknown 7). These samples were also ran according to the method conditions in table 2.
If the impurities have been correctly identified we would expect to see an increase in the relevant peak areas of the fortified samples compared to the eugenol TGAI sample.
Overlaid chromatograms of the eugenol TGAI sample and the fortified eugenol TGAI sample (spiked with impurities) are shown in figures 4 and 5.
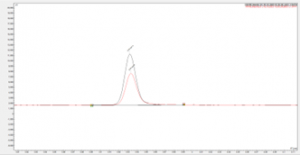
Figure 4 Chromatogram showing overlay of peak for unknown 3 in a eugenol TGAI sample and fortified eugenol TGAI sample
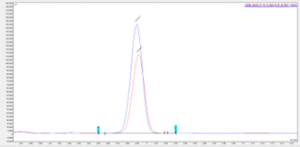
Figure 5 Chromatogram showing overlay of peak for unknown 7 in a eugenol TGAI sample and fortified eugenol TGAI sample
The fortified samples show a clear increase in the peak area of the target analytes, with an exact match in retention time, this along with the data from the NIST spectral library search shows successful identification of the significant impurities present within the eugenol TGAI batch.
The next stage after the significant impurities have been successfully identified would be a validation and assay of the relevant impurity (methyl eugenol) along with the identified significant impurities an example of this testing can be found in our application note AN166 Impurity validation and assay by GC-FID with hydrogen carrier gas.
A method validation and assay should also be performed for the active ingredient (AI), an example of this testing can be found in our application note AN164 plant protection products active ingredient assay GC-FID with nitrogen carrier gas.
Conclusion
Impurities are an important part of regulatory testing across a variety of industries. In this application note we have seen how to screen for impurities in a plant protection product or pesticide in accordance with EU regulations.
Regulations will define relevant impurities for a product and define a limit above which each significant impurity must be identified and quantified in order for successful submission of the product for use under this particular regulation.
SCION instruments recommends checking with local regulatory authorities to ensure all testing and reporting requirements are met, or contact the SCION applications team for assistance.
The principles used in this application note can be applied to not only insecticides, but to other environmental pesticidal products such as biocides and fungicides as well as across other industries such as cosmetics, food, and drug testing.
Through quantification against an analytical reference standard of the active ingredient (eugenol) we were able to determine which impurities were significant. Then by using GC-MS in tandem with the powerful NIST library search tool we were able to identify these components. Finally the identity of the impurities was confirmed using analytical reference standards.
This application note is part of a series of 3, for active ingredient method validation and assay see application note AN164, for impurity method validation and assay see application note AN166.
Download The Application Note For Ordering Information
Follow the link to download the application note and view the ordering information for the 8300 GC: Plant Protection Products Impurity Screening by GC-FID with GC-MS Confirmation
For more information, contact us.
References
- European Commission SANCO/3030/99 rev.5, 22 March 2019 https://food.ec.europa.eu/system/files/2019-03/pesticides_ppp_app-proc_guide_phys-chem-ana_3030.pdf
- Commission implementing regulation (EU) No 546/2013, Approval of Eugenol for use as a PPP, 14 June 2013 lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:163:0017:0020:EN:PDF
- Commission Implementing Regulation (EU) NO 546/2013 14 June 2013 https://www.legislation.gov.uk/eur/2013/546/2020-01-31?view=plain
