Analysis of dioxins by GC-TQMS
Introduction
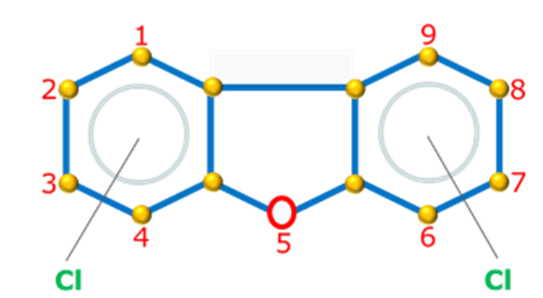
Dioxins refers to polychlorinated dibenzo-p-dioxins (PCDDs) and related compounds polychlorinated dibenzofurans (PCDFs) also called as “furans” (Figure 1). These two groups of compounds are among the most toxic chemicals and are classified as Persistent Organic Pollutants (POPs).
Figure 1. Dioxins and Furans
Dioxins are anthropogenic compounds produced unintentionally as by-products in some industrial activities and waste combustion processes, mainly. In addition to be present in the environment, dioxins are fat-soluble and bio-accumulative compounds in the tissues of animals and humans. Consumption of food is the more important exposure for humans, in particular, fish, shellfish, dairy products and meat.
Dioxins can invoke serious health effects in humans such as loss of body weight, hormone disruption, reproductive disorders, skin toxicity, immune system disorders and cancer, among other diseases. Since 2014, GC-MS/MS is an EU-accepted technique as confirmation method for dioxin analysis in accordance with EU 589/2014 and subsequent regulations implemented in this regard.
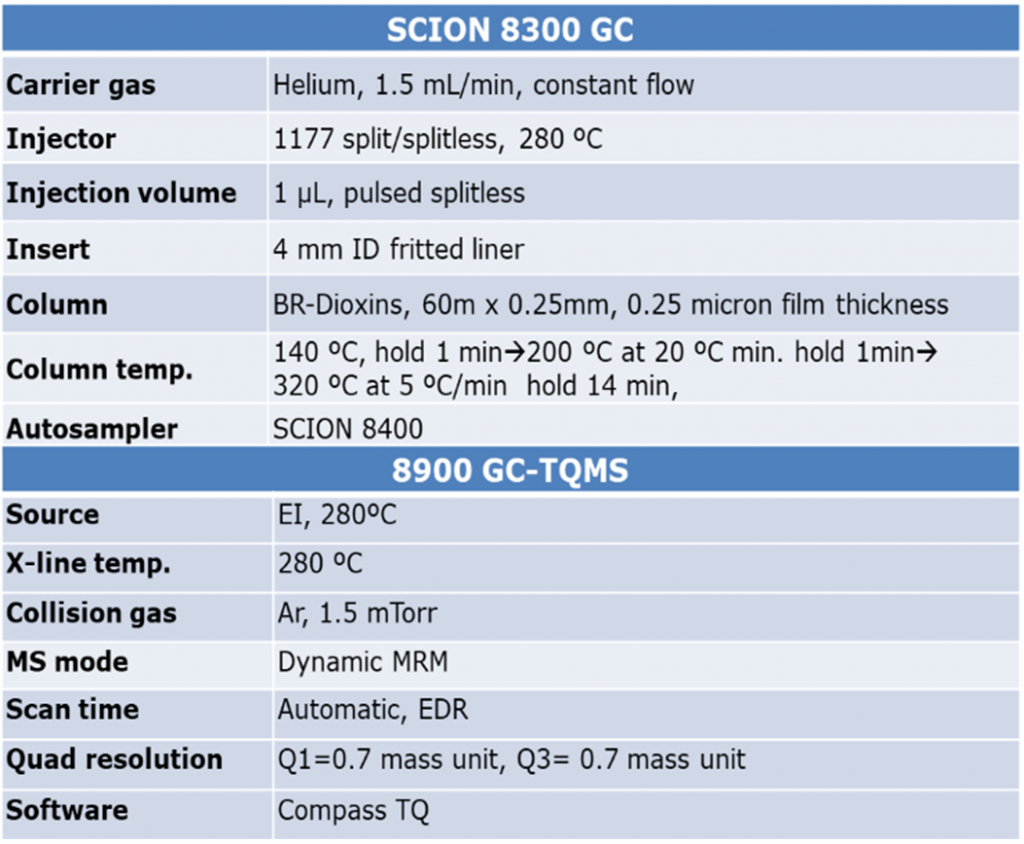
Figure 2. SCION Instruments 8300 GC coupled to a 8900 TQMS with a 8400 Autosampler
Regulations
GC-HRMS of dioxins in food and environmental samples has been the approved technique for most regulatory bodies due to high selectivity and sensitivity.
More recently, triple quadrupole MS instruments have demonstrated the improved sensitivity and selectivity needed for ultra-trace analysis of dioxins in complex matrices. In addition, GC-TQ instruments require minimal tuning and have lower maintenance requirements, are easier to operate and offer fewer chromatography interferences compared to GC-HRMS.
Therefore, since 2014, GC-MS/MS is an EU-accepted technique as a confirmation method for dioxin analysis in accordance with EU 589/2014 and subsequent regulations.
TQ GC-MS Specifications
Table 1. GC-TQMS Configuration for Dioxin Analysis
Experimental
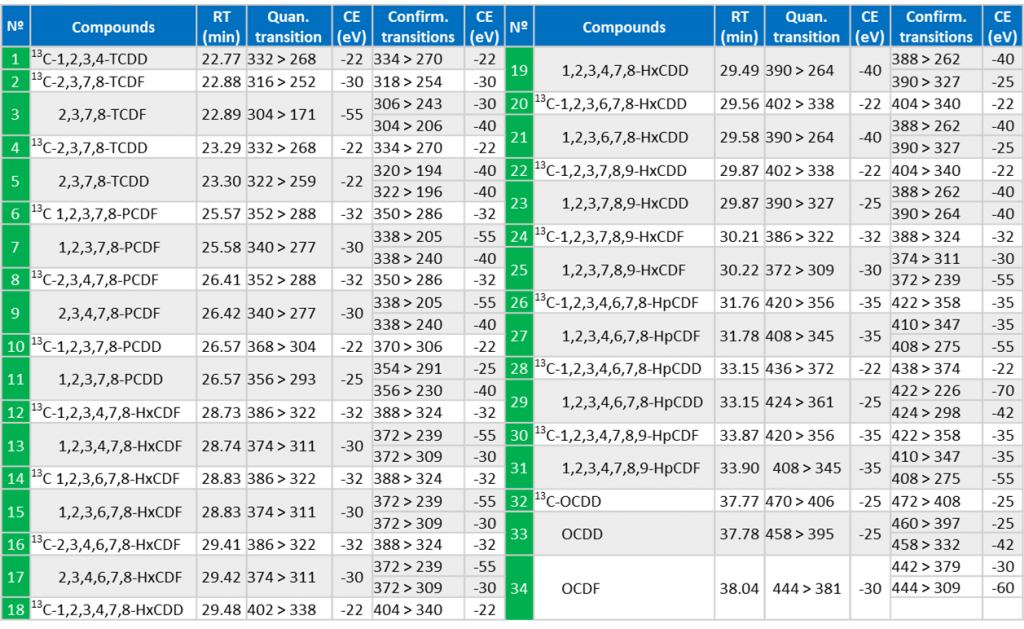
Dioxins are the only group of compounds losing COCl fragment (63 Da). This is a very selective transition producing clean MRM chromatograms with very few interferences.
Two precursor ions are selected for each compound, each with one specific MRM product ion.
Table 2: MRM transitions for dioxin analysis by GC-TQMS
Results and discussion
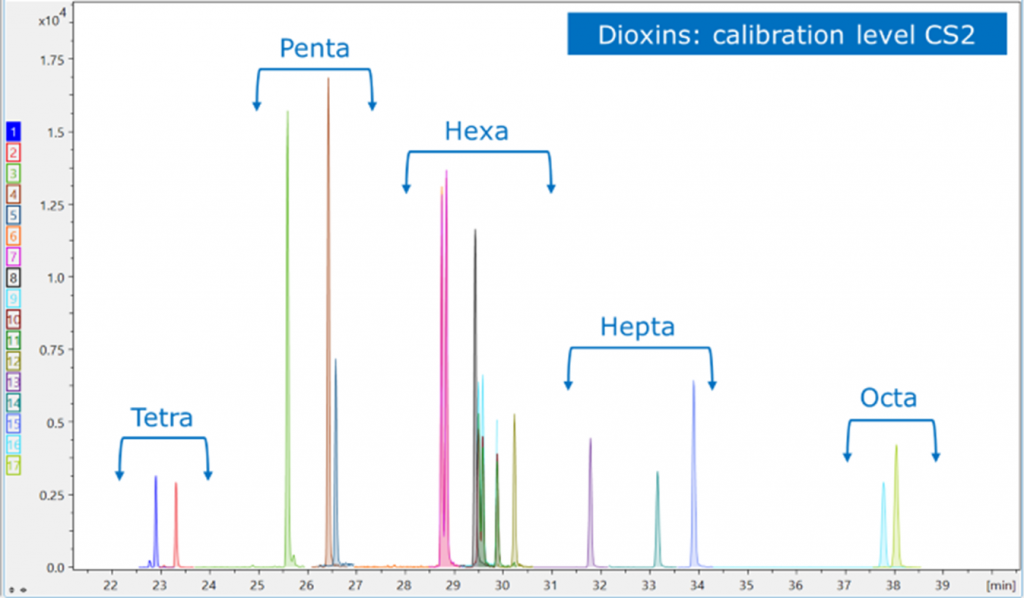
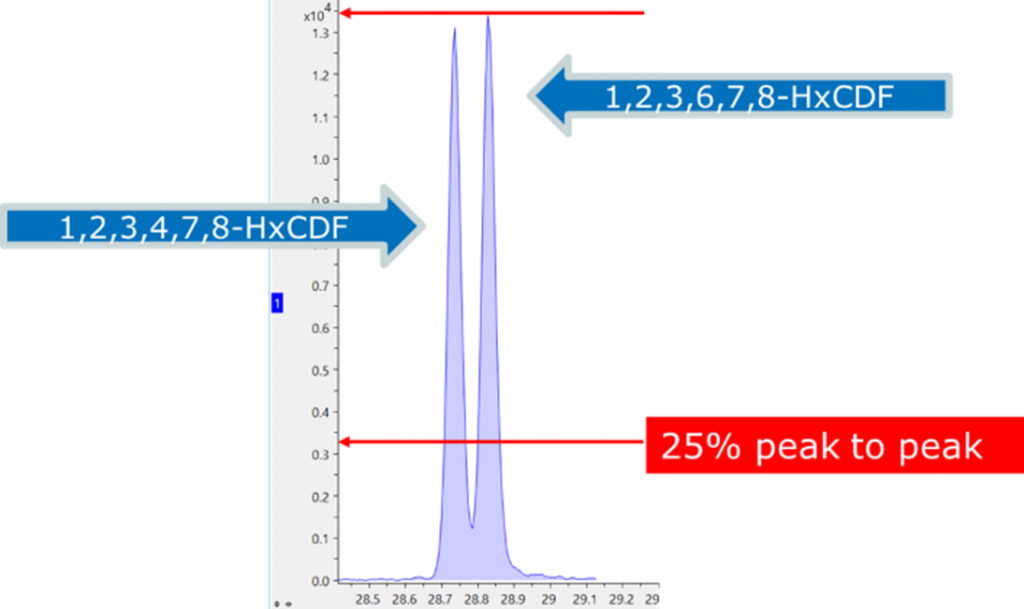
The SCION instruments 8900 TQMS is capable of resolving the different classes of dioxins using the power of MRM (Figure 3). Further, the power of the 8300 GC Is evidenced by the chromatographic resolution of the HxCDF isomers, well below the 25% peak to peak separation stipulated in the regulations for quantification (Figure 4).
Figure 3. MRM resolution of dioxin classes
Figure 4. Chromatographic resolution of dioxin isomers
The inclusion of stable isotope labelled internal standards is a key part of the regulatory framework. The Compass TQ software package for automatic quantitation allows a fast and easy overview of the results. Users can set an extensive number of evaluation rules to meet any stringent analytical regulation. These rules are applicable to any compound in the method and values can be customized by compound.
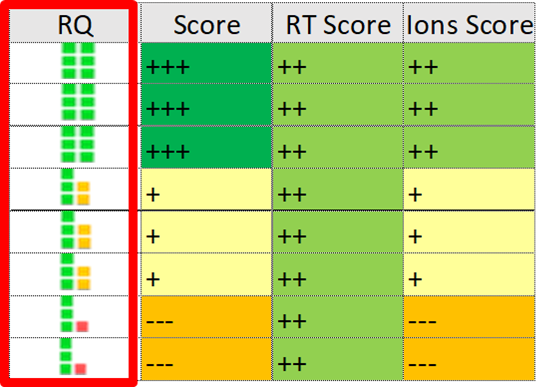
All analytical performance criteria described in EU 644/2017 have been included in the method. If the reported values are higher than the setpoint, software will trigger a red flag to warn user that the compound needs to be reviewed (Figure 5).
Figure 5. Easy visualisation of scoring
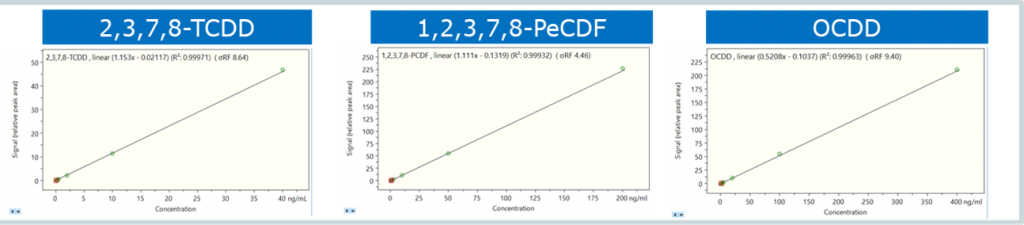
The calibration curves (Figure 6) yielded a R2 value > 0.99 with a RSD of < 13%. Retention time drift was low (<±0.1m) with recovery ranging from 94-107%.
To experimentally determine signal-to-noise ratio (s/n), the lowest calibration level CSL was diluted 10-fold and 20-fold with n-nonane. 1mL of each was injected. Both ions (quan+qual) were detected at LOQ (10fg) and LOD (5fg) levels.
Figure 6. Linearity of response
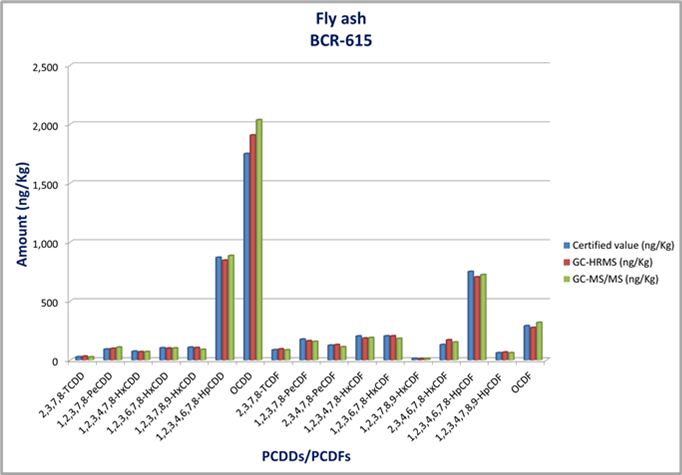
Analysis of Fly Ash
To validate the method, several commercially available certified reference materials were analyzed. Reference materials were split in two aliquot parts: one analyzed by 8900 GC-TQMS and the other sent to an external laboratory for analysis by GC-HRMS.
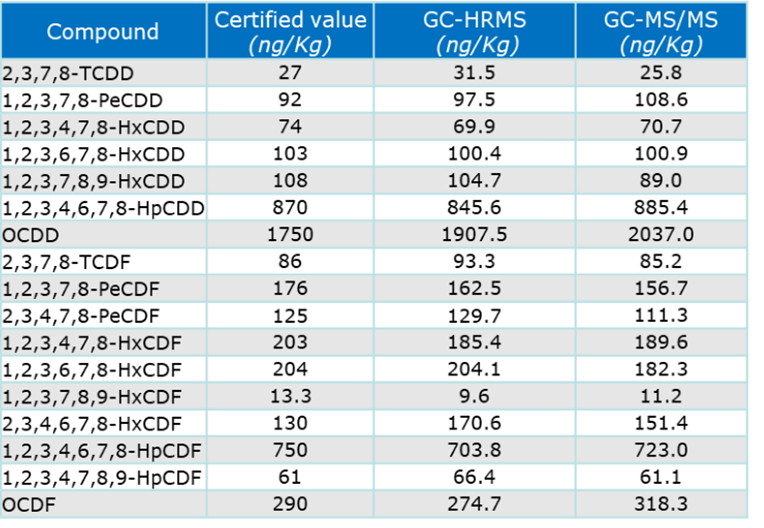
One such comparative analysis was of fly ash (Certified material BCR-615 ) . The data (Table 3, Figure 7) showed an accuracy of ± 20% with good corelation between the HRMS and TQMS results.
Table 3. Results of key compound quantification by HRMS and TQMS compared to certified values
Figure 7. Corelation of HRMS, TQMS and certified values
Determination of Dioxins in Animal Feed
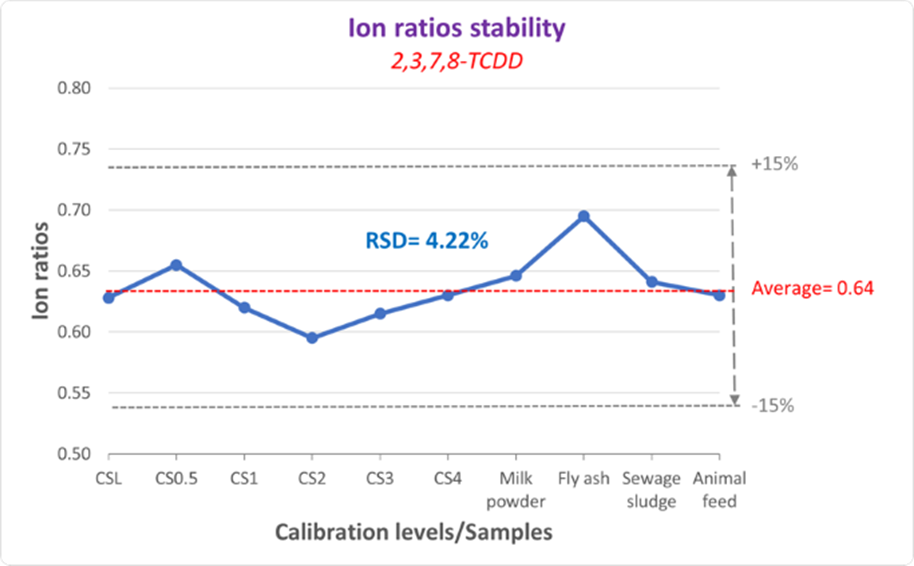
To check performance of the method in real samples, an animal feed sample was doped with dioxins at very low levels. Recovery rates of stable isotope labelled internal standards was 60-120%. For every analysis, Compass TQ software checks ion ratios tolerance (RQ score) for each target compound allowing the user to have confidence in compliance with regulatory standards. Ion ratio QC was found to have a tolerance of ± 15%. Figure 8 shows the stability of the ion ratios for 2,3,7,8-TCDD once the complete batch performed for calibration standards and samples is completed.
Figure 8. Stability of ion ratios across sample set
Conclusion
Analytical performance obtained with 8900 GC-TQMS demonstrate that this is a powerful instrument to analyze dioxins in food, feed and environmental samples with confidence. High selectivity with specific MRM transitions in addition to extensive QC parameters allows unambiguous identification and confirmation of dioxins avoiding false positives/negatives. Superb sensitivity at lower femtogram on-column level equivalent to low pg/g-TEQ levels in original samples, injecting a small sample volume (1 µL).
Download Application Note
Download complete Application Note here: Analysis of dioxins by GC-TQMS
Keep in Touch
If you wish to keep up to date with SCION Instruments latest research and articles, why not join us on social media and sign up to our newsletters today?