GC Column Selection Guide
GC Column Selection
In Gas Chromatography (GC) the column has the greatest effect on the separation of components, therefore choosing the optimal column for your analysis is essential.
The column allows for compounds in a mixture to be separated based on their boiling point and/or polarity. There are several factors to consider when choosing a column, such as application, compounds of interest, GC column type and dimensions.
1. GC column type
Generally, there are two main GC column types, packed and capillary columns. Packed columns have been since the introduction of gas chromatography in the 1950’s. These columns have a small length and large internal diameter compared to the capillary columns, and the tube is fully packed with stationary phase. The performance is relatively poor and causes peak broadening. However, they have a higher sample capacity and are still in use currently often for official analytical methods and for gas analysis.
Capillary columns were developed in the late 1950’s but first became popular during the 1980’s. Capillary columns have a significantly smaller internal diameter and tend to be much longer than packed columns. The stationary phase is coated on the inner walls of the column, making it a hollow narrow tube. This type of column shows a better resolution, sharp peaks and requires a low volume sample. Also, they can separate polar samples as well.
2. Stationary phase
Choosing the column phase represents an important step for the analysis as it makes up the fundamental of the chromatography principle. During analysis, compounds are adsorbed from the carrier gas onto the stationary phase through different chemical and physical interactions. Distinct interactions could take place, depending on the phase type, polarity and functional groups of the targeted compounds. However, a basic chemistry principle could be followed when choosing the column phase, “likes dissolves like”. Therefore, non-polar compounds are best separated on columns containing a non-polar phase and vice-versa.
When it comes to the interactions between the stationary phase and the analytes, non-polar compounds tend to present dispersive interactions with the stationary phase. They are intermolecular interactions which increase with the size of the analyte. Thus, the bigger the molecule, the longer will be retained on the column. Besides dispersive interactions, polar compounds could form other Van der Waals interactions, such as, hydrogen bonds, dipole, pi-pi and acid-base interactions due to their different functional groups that they could contain; hydroxyl, carboxyl, carbonyl, amines and so on.
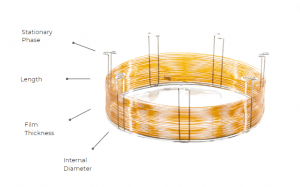
3. Column length
Choosing the column length is important as well, it balances resolution and analysis time. Long columns provide substantial resolution, but it increases the back pressure. Also, resolution is not proportional to column length, meaning that doubling the column length will not double the resolution. Increasing column length should be the last resort taken to improve resolution. A better way to do it is to decrease the internal diameter. Regarding short columns, the resolution provided is poor, and they should be used for qualitative analysis only. However, this can be counterbalanced by having a small internal diameter as well. This has been shown to preserve resolution, and in some cases to even increase it.
4. Column internal diameter
The column internal diameter deals with the number of theoretical plates, so the efficiency of the separation. The smaller the internal diameter, the higher the number of theoretical plates which increases efficiency. However, the sample load is significantly decreased due to the narrow diameter and low flow rate should be used. Exceeding the column capacity to accommodate the analytes will display poor resolution and asymmetric peaks. When choosing the internal diameter, a balance between efficiency and sample load is made.
5. Column film thickness
Another parameter to be considered is the film thickness of the column. Depending on the application, a decreased or increased film thickness might be used. It is optimal to use a low film thickness to obtain a better resolution by sharpening the peak. Also, the maximum temperature limit is higher as well and the analysis time shorter. However, by having a decreased film thickness enhances the interaction of the analytes with the tube wall and decreases sample capacity.
By increasing the film thickness, the interaction between the analyte and the tube is significantly reduced, high concentration samples could be easily analyzed, and the capacity factor parameter is increased. Also, low boiling point compounds are best to be analyzed with a column having a thicker film. Still, due to the larger amount of stationary phase, the column bleed is more readily increased, and the maximum allowed temperature will be lower.
Get In Touch
If you have a specific consumables need that hasn’t been discussed in this introduction, please explore our spares and consumables and/or get in touch with us or your local SCION sales representative for assistance.

