New release of CompassCDS Chromatography Data System from SCION Instruments
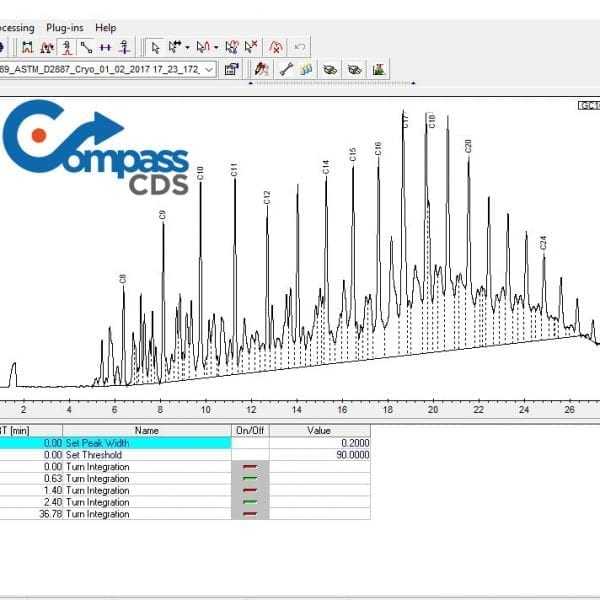 SCION Instruments, a global supplier of GC, GCMS and Chromatography Data Systems is pleased to announce release of CompassCDS version 4.1 with new features and capabilities which extend its applicability to a wider range of laboratories.
SCION Instruments, a global supplier of GC, GCMS and Chromatography Data Systems is pleased to announce release of CompassCDS version 4.1 with new features and capabilities which extend its applicability to a wider range of laboratories.
Built on the history of Varian and Bruker, CompassCDS brings more than 20 years of customer experience and development to your desktop. It provides a user-friendly and application-rich GUI with unique integration and extended calculation and reporting capabilities. Additionally, industry-, function-, and application-specific add-ons and plug-ins are available to fit a wide range of specific laboratory needs. Robust in design for 24/7 operations it scales from stand-alone to enterprise-wide client/server installations with centralized system administration and data management. CompassCDS also complies to national and international regulations and guidelines including 21CFR11 and ISO/IEC 17025.
New features in CompassCDS 4.1 include:
- Support of Agilent’s Instrument Control Framework (ICF)
- RC.Net control drivers for Agilent LC 1100, 1120/1220 Compact, 1200 and 1260 Infinity series, and 1260 and 1290 Infinity II series
- RC.Net control drivers for Agilent GC 9000 Intuvo, 7890 A/B and 7820 including 7697A and G1888 A headspace autosamplers
- RC.Net control drivers for CTC PAL RTC, RSI and LSI
- Updated AnIML converter and viewer for long-term archiving of raw data, meta data and results
Together, these additions in Compass CDS 4.1 extend the applicability of Compass into Pharma/Biotech, Food safety and Chemical laboratories.
For more details on SCION Instruments CompassCDS and GC solutions, as well as service and support on new and legacy systems, please contact SCION Instruments using the contact details on the SCION Instruments web site.
